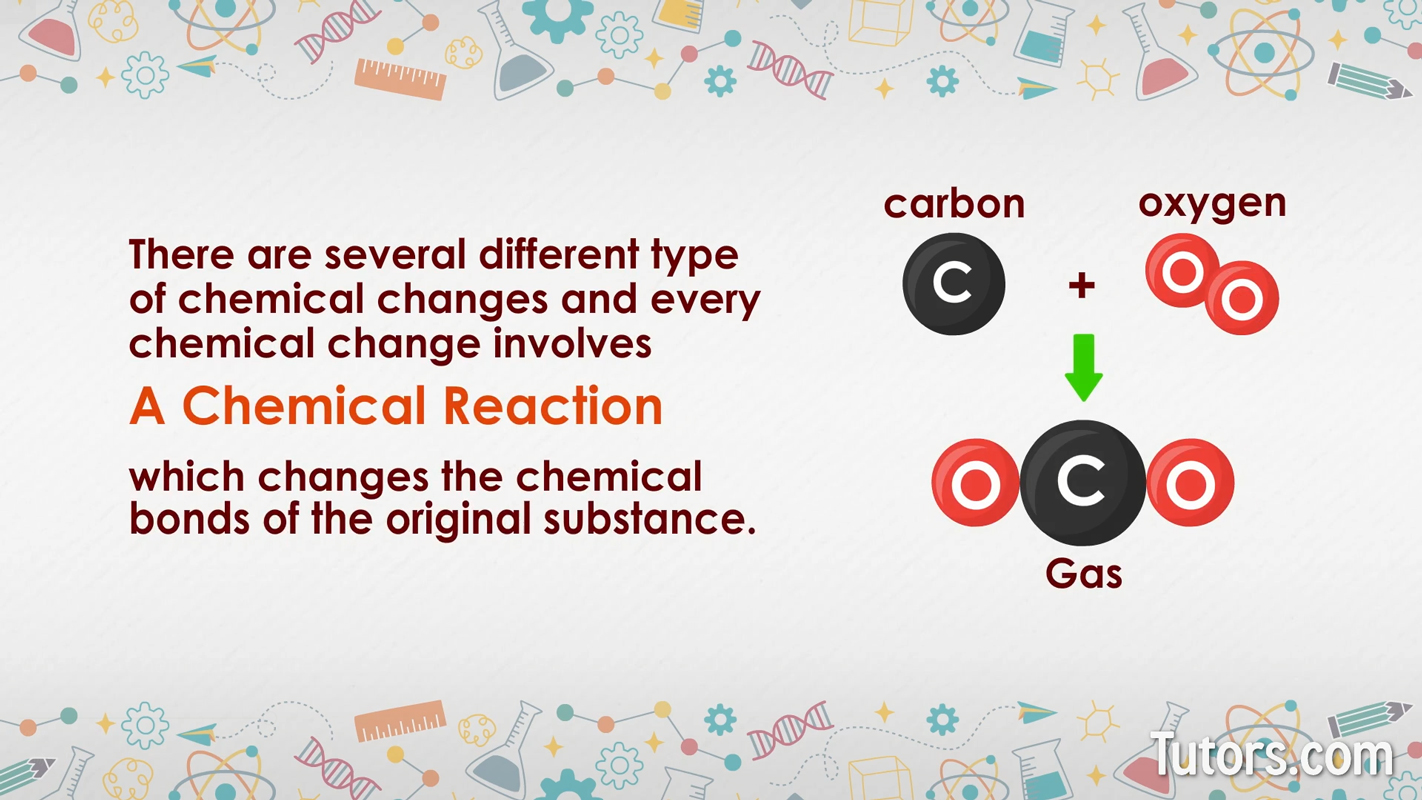
A chemical change is a reaction that alters the chemical composition of a pure substance. They occur when a pure substance becomes one or more different pure substances. There are several types of chemical changes.
Every chemical change involves a chemical reaction, which changes the chemical bonds of the original substance. Chemical reactions create one new chemical substance or several new substances (the products) from one or more reactants.


Observing only one reaction is not enough to know you have a chemical change and not a physical change.
Expect to see several characteristics from this list of seven signs of a chemical change.
Get free estimates from chemistry tutors near you.We see many types of chemical changes all around us and even within our bodies.
Here are eight types of chemical reactions:
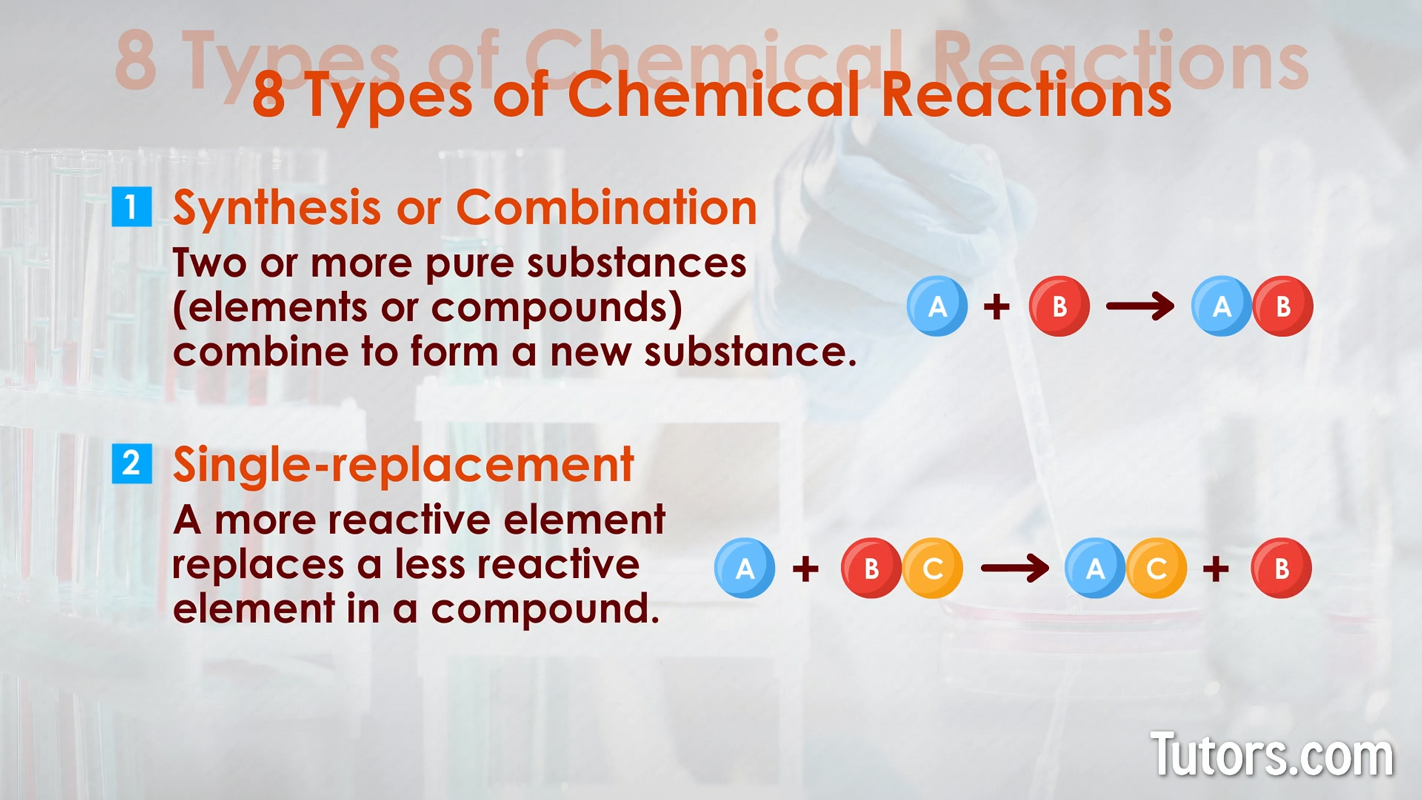
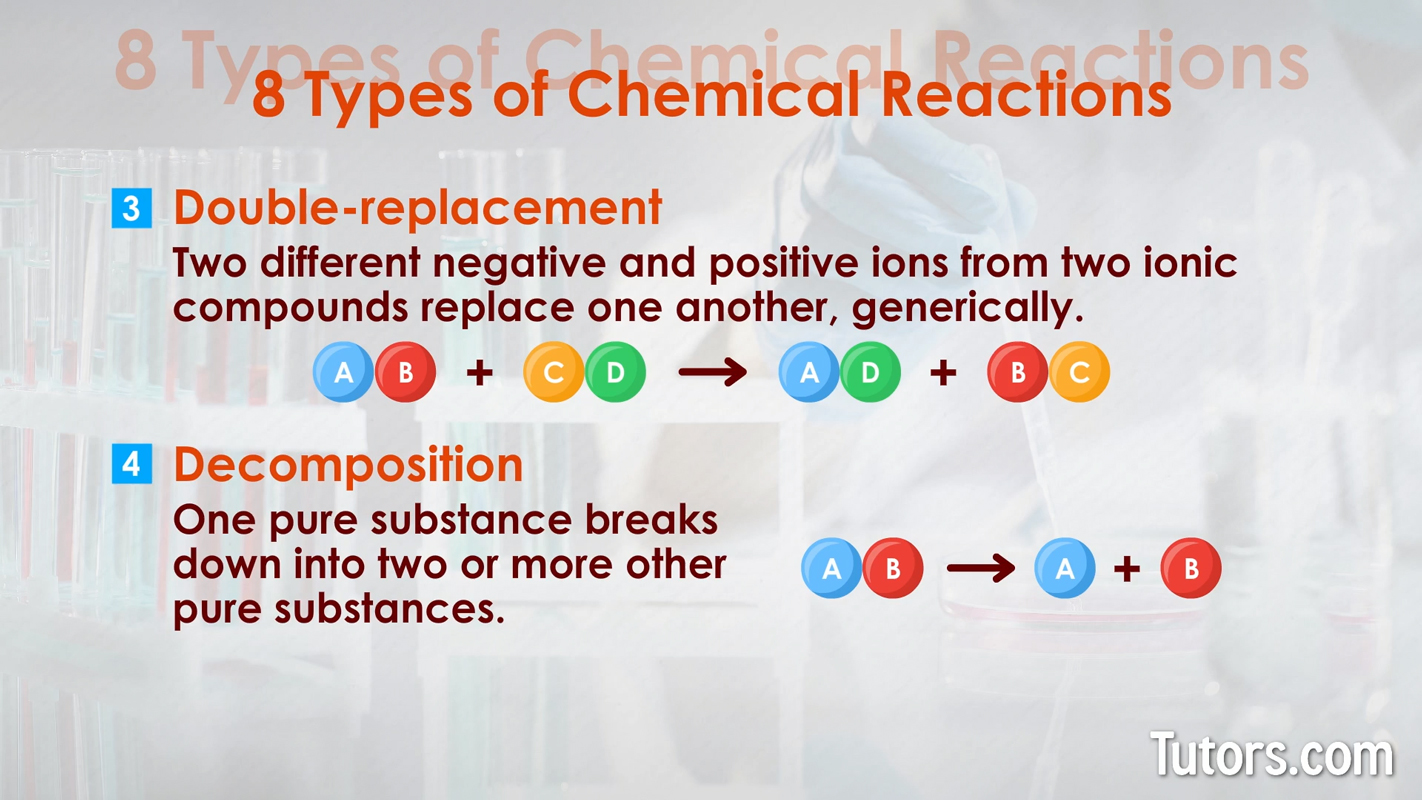
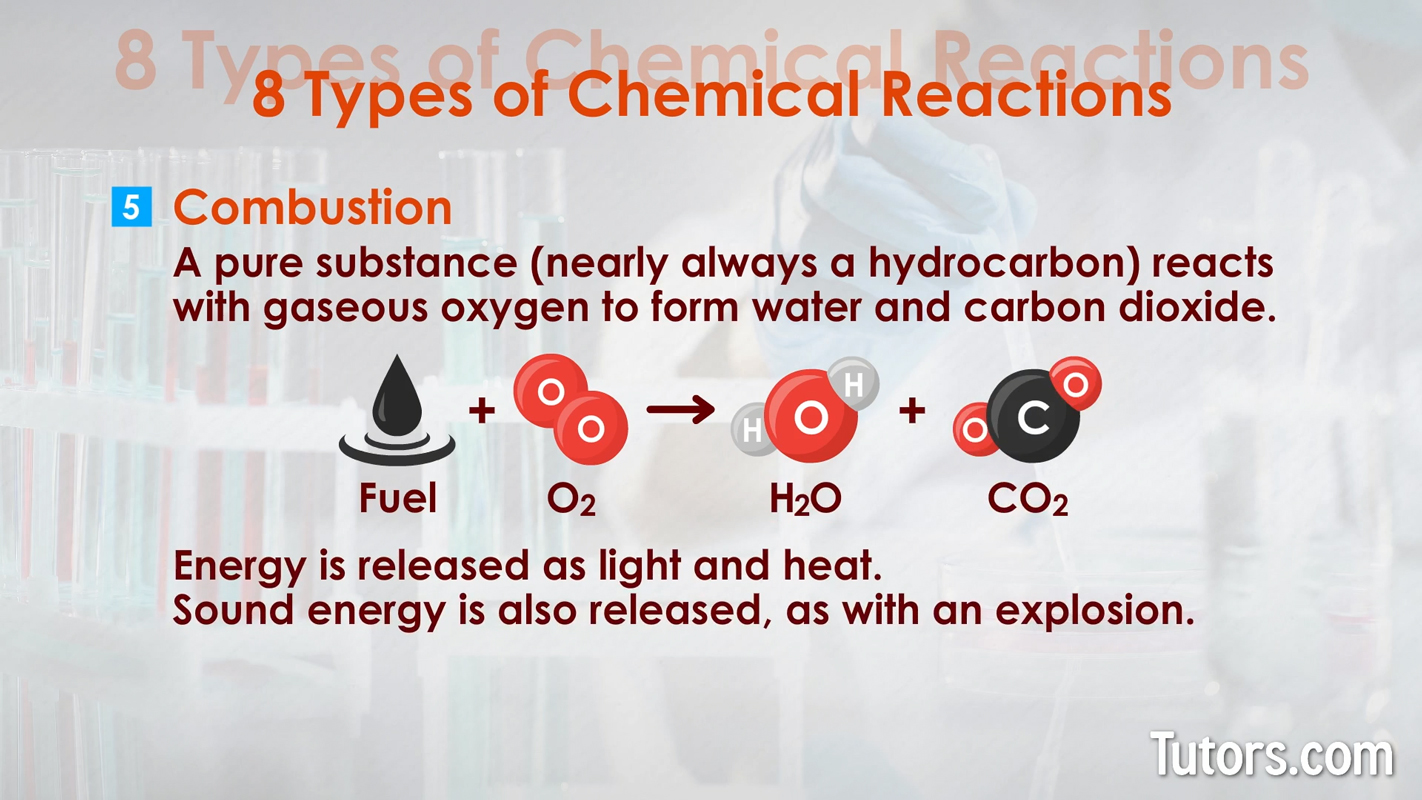
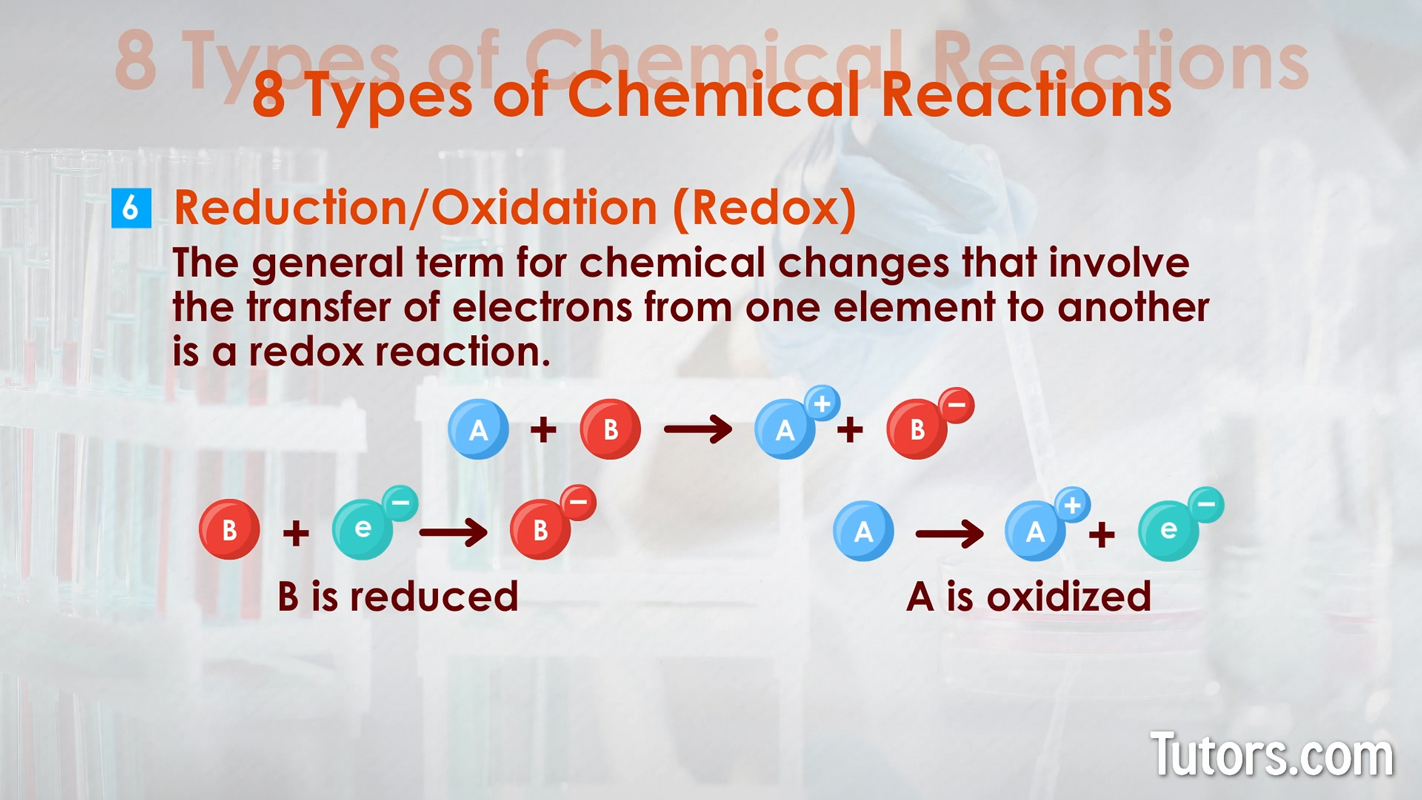
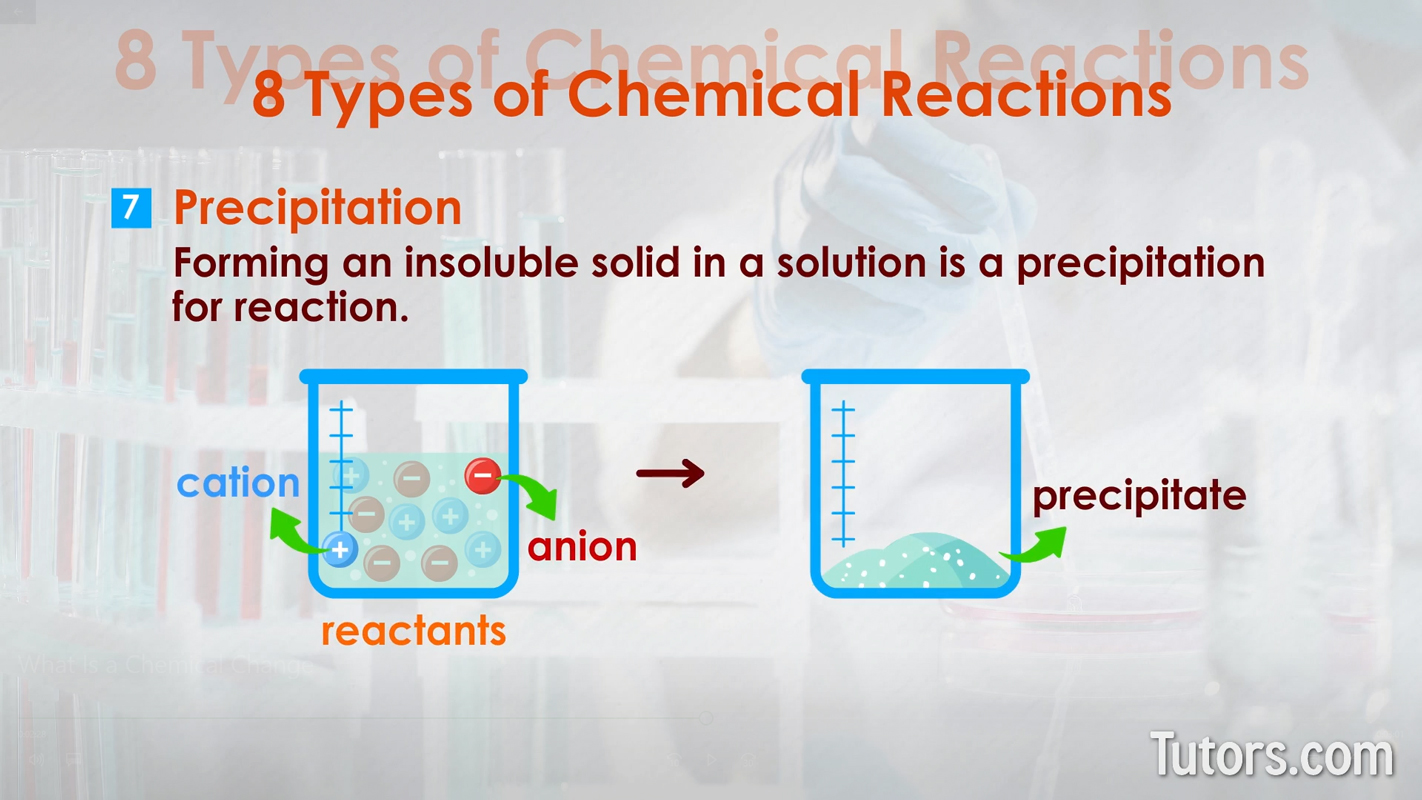
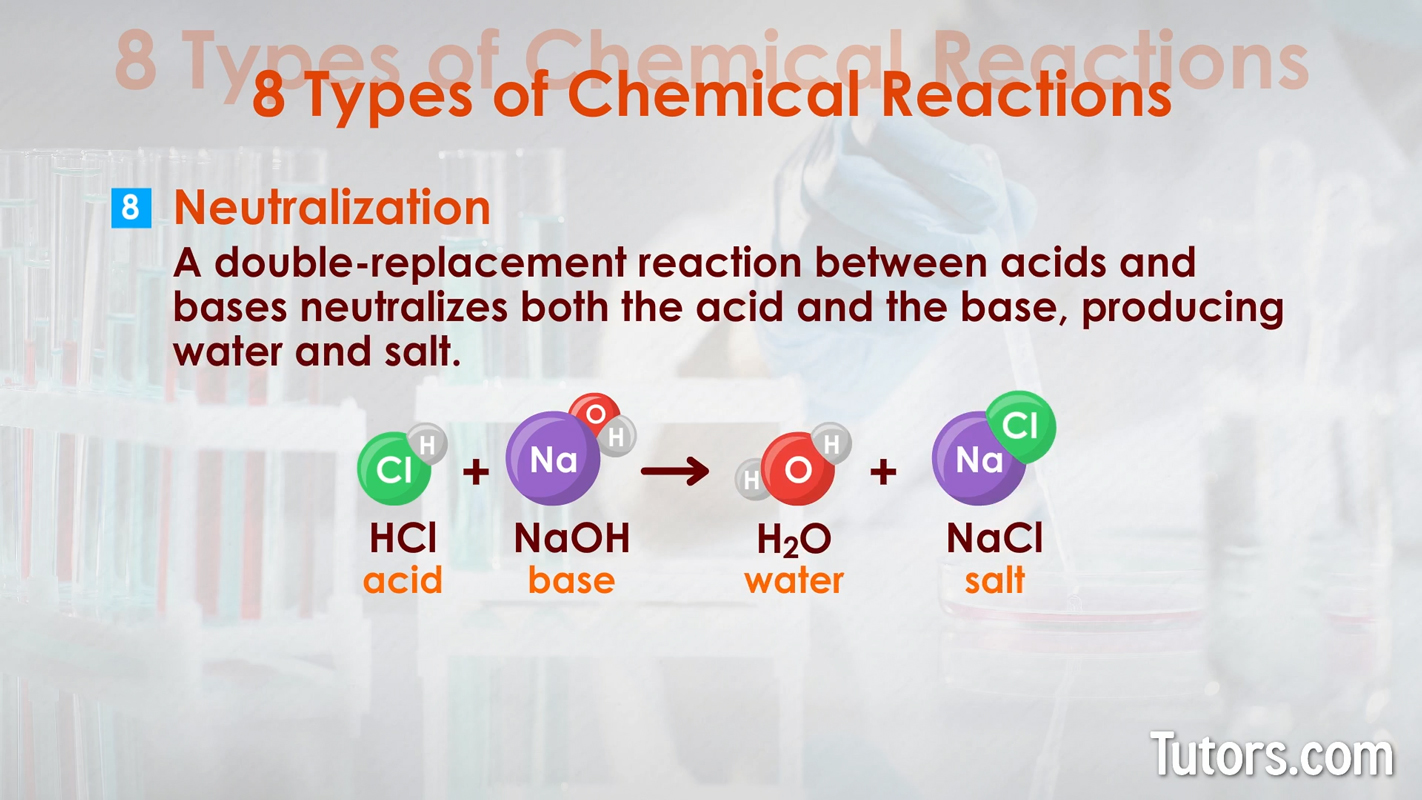
Chemical changes can occur quickly, as with combustion. Chemical changes can occur slowly, as with iron rusting into iron oxide or your body growing from a baby to an adult.
Imagine that you receive an unwelcome note on a scrap of paper from a classmate. What can you do? Burn it, cut it up, fold it into a paper airplane?
You can do a lot of physical and chemical things to a sheet of paper.
Burning the unwanted paper is an example of a chemical change.
You cannot get back the original paper because you destroyed the paper itself. No matter what you try, you cannot recover the paper and the writing.
For example, an explosion is a combustion reaction that will produce heat, light, sound, gas, a change in color, and a temperature change.
When oxygen ( O 2 _ O 2 ) reacts with fuel, it chemically changes to carbon dioxide ( C O 2 C_ C O 2 ) and water ( H 2 O _O H 2 O ) while releasing energy. That is a combustion reaction; it is a chemical change.
Chemical changes in biology are known as biochemical changes. Two examples of biochemical changes include fermentation and photosynthesis.
The single most important result of a chemical change is the permanent transformation of the original substance. Chemically changing a substance produces a completely different substance - the matter changes the chemical makeup completely.
The products from the chemical change will also have almost no chemical properties or physical properties in common with the reactants. That piece of paper was cellulose; now, it is ash (carbon and some other compounds). The paper (the reactant) will never return.
Iron is magnetic, while iron sulfide that results from the synthesis of iron and sulfur is not magnetic. All chemical changes are one-way changes. Old substances transform into new substances.
Chemistry and physics are better understood when you know how to classify physical and chemical changes. If you alter the shape, size, or state of matter of some object but still have the object itself (in some form), you have performed a physical change.

Changes in states of matter include four physical changes for the four states of matter (solids, liquids, gases, and plasmas).
Specific names for these changes apply to liquids and solids, solids and gases, gases and liquids, and gases and plasmas.
Here is a list of reactions that physically change the state of matter; each one has its opposite:
With all physical changes, you can get back the original substance. Some physical changes are harder to reverse than others, but all physical changes can be “undone.”
Melting an ice cube may show a change in temperature, but that is only one of eight signals, which is not enough to say that melting is a chemical change.